2009 flu pandemic
| Pandemic H1N1/09 Influenza | |
|---|---|
| Classification and external resources | |
 Electron microscope image of the reassorted H1N1 influenza virus. The viruses are ~100 nanometres in diameter.[1] |
|
| MedlinePlus | 007421 |
| eMedicine | article/1673658 |
| MeSH | D053118 |
| Influenza (Flu) |
|---|
 |
| Types |
| Avian (A/H5N1 subtype) · Canine Equine · Swine (A/H1N1 subtype) |
| Vaccines |
| 2009 pandemic (Pandemrix) ACAM-FLU-A · Fluzone · Influvac Live attenuated (FluMist) · Optaflu |
| Treatment |
| Amantadine · Arbidol · Laninamivir Oseltamivir · Peramivir · Rimantadine Vitamin D · Zanamivir |
| Pandemics |
| 2009 · 1968–1969 Hong Kong · 1918 |
| Outbreaks |
| 2008 West Bengal 2007 Bernard Matthews H5N1 2007 Australian equine 2006 H5N1 India · 1976 swine flu |
| See also |
| Flu season · Influenza evolution Influenza research Influenza-like illness |
The 2009 flu pandemic was a global outbreak of a new strain of H1N1 influenza virus, often referred to as "swine flu".[2] First described in April 2009, the virus appeared to be a new strain of H1N1 which resulted when a previous triple reassortment of bird, pig, and human flu viruses further combined with a Eurasian pig flu virus.[3] Unlike most strains of influenza, H1N1 does not disproportionately infect adults older than 60 years; this was an unusual and characteristic feature of the H1N1 pandemic.[4] Even for persons previously very healthy, a small percentage of patients will develop viral pneumonia or acute respiratory distress syndrome. This manifests itself as increased breathing difficulty and typically occurs 3–6 days after initial onset of flu symptoms.[5][6]
The outbreak began in the state of Veracruz, Mexico, with evidence that there had been an ongoing epidemic for months before it was officially recognized as such.[7] The Mexican government closed most of Mexico City's public and private facilities in an attempt to contain the spread of the virus, however the virus continued to spread globally, and clinics in some areas were overwhelmed by people infected. In June, the World Health Organization (WHO) and US Centers for Disease Control (CDC) stopped counting cases and declared the outbreak to be a pandemic.[8]
Despite being informally called "swine flu", the H1N1 flu virus cannot be spread by eating pork or pork products;[9][10] similar to other influenza viruses, it is typically contracted by person to person transmission through respiratory droplets.[11] Symptoms usually last 4–6 days.[12] Antivirals (oseltamivir or zanamivir) were recommended for those with more severe symptoms or those in an at-risk group.[13] Currently, there are 14,286 confirmed deaths worldwide. This figure is a sum of confirmed deaths reported by national authorities; the WHO states that total mortality (including deaths unconfirmed or unreported) from the new H1N1 strain is "unquestionably higher".[14]
The pandemic began to taper off in November 2009,[15] and by May 2010, the number of cases was in steep decline.[14][16][17][18] On 10 August 2010, the Director-General of the World Health Organization, Margaret Chan, announced the end of H1N1 pandemic.[19] Chan noted that the H1N1 pandemic could have been much worse. According to the latest World Health Organization statistics, the virus has killed more than 18,000 people since it appeared in April 2009,[20] approximately 4% of the 250,000 to 500,000 annual influenza deaths.[21] Critics claimed the WHO had exaggerated the danger, spreading "fear and confusion" rather than "immediate information."[22] The WHO began an investigation to determine[23] whether it had "frightened people unnecessarily."[24]
Contents |
Classification
The initial outbreak was called the "H1N1 influenza", or "Swine Flu" by American media. It is called pandemic H1N1/09 virus by the WHO,[25] while the CDC refers to it as "novel influenza A (H1N1)" or "2009 H1N1 flu".[26] In the Netherlands, it was originally called "Pig Flu", but is now called "New Influenza A (H1N1)" by the national health institute, although the media and general population use the name "Mexican Flu". South Korea and Israel briefly considered calling it the "Mexican virus".[27] Later, the South Korean press used "SI", short for "swine influenza". Taiwan suggested the names "H1N1 flu" or "new flu", which most local media adopted.[28] The World Organization for Animal Health proposed the name "North American influenza".[29] The European Commission adopted the term "novel flu virus".[30]
Signs and symptoms
The symptoms of H1N1 flu are similar to other influenzas, and may include a fever, cough (typically a "dry cough"), headache, muscle or joint pain, sore throat, chills, fatigue, and runny nose. Diarrhea, vomiting, and neurological problems have also been reported in some cases.[31][32] People at higher risk of serious complications include those aged over 65, children younger than 5, children with neurodevelopmental conditions, pregnant women (especially during the third trimester),[5][33] and those of any age with underlying medical conditions, such as asthma, diabetes, obesity, heart disease, or a weakened immune system (e.g., taking immunosuppressive medications or infected with HIV).[34] More than 70% of hospitalizations in the US have been people with such underlying conditions, according to the CDC.[35]
In September 2009, the CDC reported that the H1N1 flu "seems to be taking a heavier toll among chronically ill children than the seasonal flu usually does."[36] Through August 8, 2009, CDC had received 36 reports of pediatric deaths with associated influenza symptoms and laboratory-confirmed pandemic H1N1 from state and local health authorities within the United States, with 22 of these children having neurodevelopmental conditions such as cerebral palsy, muscular dystrophy, or developmental delays.[37] "Children with nerve and muscle problems may be at especially high risk for complications because they cannot cough hard enough to clear their airways."[36] From April 26, 2009, to Feb. 13, 2010, CDC had received reports of the deaths of 277 children with laboratory-confirmed 2009 influenza A (H1N1) within the United States.[38]
Symptoms in severe cases
The World Health Organization reports that the clinical picture in severe cases is strikingly different from the disease pattern seen during epidemics of seasonal influenza. While people with certain underlying medical conditions are known to be at increased risk, many severe cases occur in previously healthy people. In these patients, predisposing factors that increase the risk of severe illness are not presently understood, though research is under way. In severe cases, patients generally begin to deteriorate around three to five days after symptom onset. Deterioration is rapid, with many patients progressing to respiratory failure within 24 hours, requiring immediate admission to an intensive care unit. Upon admission, most patients need immediate respiratory support with mechanical ventilation.[39]
A November 2009 CDC recommendation stated that the following constitute "emergency warning signs" and advised seeking immediate care if a person experiences any one of these signs:[40]
- In adults:
-
- Difficulty breathing or shortness of breath
- Pain or pressure in the chest or abdomen
- Sudden dizziness
- Confusion
- Severe or persistent vomiting
- Low temperature
- In children:
-
- Fast breathing or working hard to breathe
- Bluish skin color
- Not drinking enough fluids
- Not waking up or not interacting
- Being so irritable that the child does not want to be held
- Flu-like symptoms that improve but then return with fever and worse cough
- Fever with a rash
- Being unable to eat
- Having no tears when crying
Complications
Most complications have occurred among previously healthy individuals, with obesity and respiratory disease as the strongest risk factors. Pulmonary complications are common. Primary influenza pneumonia occurs most commonly in adults and may progress rapidly to acute lung injury requiring mechanical ventilation. Secondary bacterial infection is more common in children. Staphylococcus aureus, including methicillin-resistant strains, is an important cause of secondary bacterial pneumonia with a high mortality rate. Neuromuscular and cardiac complications are unusual but may occur.[41]
A United Kingdom investigation of risk factors for hospitalisation and poor outcome with pandemic A/H1N1 influenza looked at 631 patients from 55 hospitals admitted with confirmed infection from May through September 2009. 13% were admitted to a high dependency or intensive care unit and 5% died; 36% were aged <16 years and 5% were aged ≥65 years. Non-white and pregnant patients were over-represented. 45% of patients had at least one underlying condition, mainly asthma, and 13% received antiviral drugs before admission. Of 349 with documented chest x-rays on admission, 29% had evidence of pneumonia, but bacterial co-infection was uncommon. Multivariate analyses showed that physician-recorded obesity on admission and pulmonary conditions other than asthma or chronic obstructive pulmonary disease (COPD) were associated with a severe outcome, as were radiologically confirmed pneumonia and a raised C-reactive protein (CRP) level (≥100 mg/l). 59% of all in-hospital deaths occurred in previously healthy people.[42]
Fulminant myocarditis has been linked to infection with H1N1, with at least four cases of myocarditis confirmed in patients also infected with A/H1N1. Three out of the four cases of H1N1 associated myocarditis were classified as fulminant, and one of the patients died.[43] Also, there appears to be a link between severe A/H1N1 influenza infection and pulmonary embolism. In one report, 5 out of 14 patients admitted to the intensive care unit with severe A/H1N1 infection were found to have pulmonary emboli.[44]
Diagnosis
Confirmed diagnosis of pandemic H1N1 flu requires testing of a nasopharyngeal, nasal, or oropharyngeal tissue swab from the patient.[45] Real-time RT-PCR is the recommended test as others are unable to differentiate between pandemic H1N1 and regular seasonal flu.[45] However, most people with flu symptoms do not need a test for pandemic H1N1 flu specifically, because the test results usually do not affect the recommended course of treatment.[46] The CDC recommends testing only for people who are hospitalized with suspected flu, pregnant women, and people with weakened immune systems.[46] For the mere diagnosis of influenza and not pandemic H1N1 flu specifically, more widely available tests include rapid influenza diagnostic tests (RIDT), which yield results in about 30 minutes, and direct and indirect immunofluorescence assays (DFA and IFA), which take 2–4 hours.[47] Due to the high rate of RIDT false negatives, the CDC advises that patients with illnesses compatible with novel influenza A (H1N1) virus infection but with negative RIDT results should be treated empirically based on the level of clinical suspicion, underlying medical conditions, severity of illness, and risk for complications, and if a more definitive determination of infection with influenza virus is required, testing with rRT-PCR or virus isolation should be performed.[48] Dr. Rhonda Medows of the Georgia Department of Community Health states that the rapid tests are incorrect anywhere from 30 to 90% of the time and warns doctors in her state not to use them because they are wrong so often.[49] The use of RIDTs has also been questioned by researcher Paul Schreckenberger of the Loyola University Health System, who suggests that rapid tests may actually pose a dangerous public health risk.[50] Dr. Nikki Shindo of the WHO has expressed regret at reports of treatment being delayed by waiting for H1N1 test results and suggests that "doctors should not wait for the laboratory confirmation but make diagnosis based on clinical and epidemiological backgrounds and start treatment early."[51]
On June 22, 2010, the CDC announced a new test called the "CDC Influenza 2009 A (H1N1)pdm Real-Time RT-PCR Panel (IVD)". It uses a molecular biology technique to detect influenza A viruses and specifically the 2009 H1N1 virus. The new test will replace the previous real-time RT-PCR diagnostic test used during the 2009 H1N1 pandemic, which received an emergency use authorization by the FDA in April 2009. Tests results are available in four hours and are 96% accurate.[52]
Virus characteristics
The virus was found to be a novel strain of influenza for which extant vaccines against seasonal flu provided little protection. A study at the US Centers for Disease Control and Prevention, published in May 2009, found that children had no preexisting immunity to the new strain but that adults, particularly those over 60, had some degree of immunity. Children showed no cross-reactive antibody reaction to the new strain, adults aged 18 to 64 had 6–9%, and older adults 33%.[53][54] While it has been thought that these findings suggest the partial immunity in older adults may be due to previous exposure to similar seasonal influenza viruses, a November 2009 study of a rural unvaccinated population in China found only a 0.3% cross-reactive antibody reaction to the H1N1 strain, suggesting that previous vaccinations for seasonal flu and not exposure may have resulted in the immunity found in the older US population.[55]
It has been determined that the strain contains genes from five different flu viruses: North American swine influenza, North American avian influenza, human influenza, and two swine influenza viruses typically found in Asia and Europe. Further analysis has shown that several proteins of the virus are most similar to strains that cause mild symptoms in humans, leading virologist Wendy Barclay to suggest on May 1, 2009, that the initial indications are that the virus was unlikely to cause severe symptoms for most people.[56]
The virus is currently less lethal than previous pandemic strains and kills about 0.01–0.03% of those infected; the 1918 influenza was about one hundred times more lethal and had a case fatality rate of 2–3%.[57] By November 14, the virus had infected one in six Americans with 200,000 hospitalizations and 10,000 deaths – as many hospitalizations and fewer deaths than in an average flu season overall, but with much higher risk for those under 50. With deaths of 1,100 children and 7,500 adults 18 to 64, these figures "are much higher than in a usual flu season".[58]
In June 2010 Scientists from Hong Kong reported discovery of a new swine flu virus that is a hybrid of the pandemic H1N1 virus and viruses previously found in pigs. It is the first report of a reassortment of the pandemic virus, which in humans has been slow to evolve. Nancy Cox, head of the influenza division at the U.S. Centers for Disease Control, has said, "This particular paper is extremely interesting because it demonstrates for the first time what we had worried about at the very onset of the pandemic, and that is that this particular virus, when introduced into pigs, could reassort with the resident viruses in pigs and we would have new gene constellations. And bingo, here we are." Pigs have been termed the mixing vessel of flu because they can be infected both by avian flu viruses, which rarely directly infect people, and by human viruses. When pigs become simultaneously infected with more than one virus, the viruses can swap genes, producing new variants that can pass to humans and sometimes spread among them.[59] "Unlike the situation with birds and humans, we have a situation with pigs and humans where there's a two-way street of exchange of viruses. With pigs it's very much a two-way street."[60]
Transmission
Spread of the H1N1 virus is thought to occur in the same way that seasonal flu spreads. Flu viruses are spread mainly from person to person through coughing or sneezing by people with influenza. Sometimes people may become infected by touching something – such as a surface or object – with flu viruses on it and then touching their face. "Avoid touching your eyes, nose or mouth. Germs spread this way."[9]
The basic reproduction number (the average number of other individuals that each infected individual will infect, in a population that has no immunity to the disease) for the 2009 novel H1N1 is estimated to be 1.75.[61] A December 2009 study found that the transmissibility of the H1N1 influenza virus in households is lower than that seen in past pandemics. Most transmissions occur soon before or after the onset of symptoms.[62]
The H1N1 virus has been transmitted to animals, including swine, turkeys, ferrets, household cats, at least one dog, and a cheetah.[63][64][65][66]
Prevention
The H1N1 vaccine was initially in short supply and the CDC recommended that initial doses should go to priority groups such as pregnant women, people who live with or care for babies under six months old, children six months to four years old and health-care workers.[67] In the UK, the NHS recommended vaccine priority go to people over six months old who were clinically at risk for seasonal flu, pregnant women, and households of people with compromised immunity.[68]
Although it was initially thought that two injections would be required, clinical trials showed that the new vaccine protects adults "with only one dose instead of two", and so the limited vaccine supplies would go twice as far as had been predicted.[69][70] Costs would also be lowered by having a "more efficient vaccine".[69] For children under the age of 10, two administrations of the vaccine, spaced 21 days apart, are recommended.[71][72] The seasonal flu will still require a separate vaccination.[73]
Health officials worldwide were also concerned because the virus was new and could easily mutate and become more virulent, even though most flu symptoms were mild and lasted only a few days without treatment. Officials also urged communities, businesses and individuals to make contingency plans for possible school closures, multiple employee absences for illness, surges of patients in hospitals and other effects of potentially widespread outbreaks.[74]
In February 2010, CDC's Advisory Committee on Immunization Practices voted for "universal" flu vaccination in the U.S. to include all people over six months of age. Next season's vaccine will protect against the 2009 H1N1 pandemic virus and two other flu viruses.[75]
Public health response
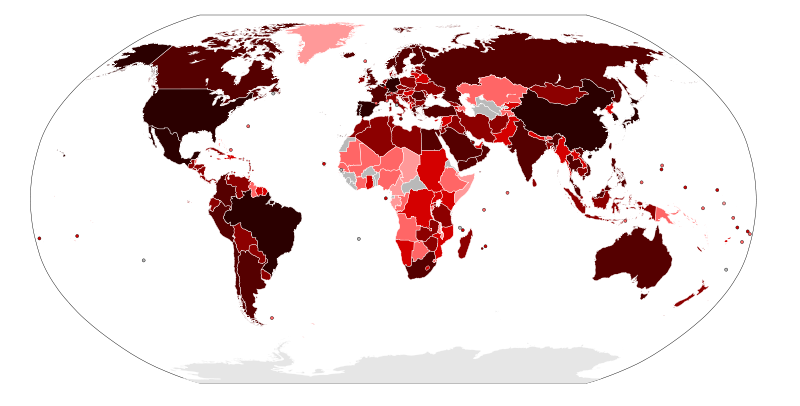
On April 27, 2009, the European Union health commissioner advised Europeans to postpone nonessential travel to the United States or Mexico. This followed the discovery of the first confirmed case in Spain.[76] On May 6, 2009, the Public Health Agency of Canada announced that their National Microbiology Laboratory (NML) had mapped the genetic code of the swine flu virus, the first time that had been done.[77] In England, the National Health Service launched a website, the National Pandemic Flu Service,[78] allowing patients to self-assess and get an authorization number for antiviral medication. The system was expected to reduce the burden on general practitioners.[68]
US officials observed that six years of concern about H5N1 avian flu did much to prepare for the current H1N1 flu outbreak, noting that after H5N1 emerged in Asia, ultimately killing about 60% of the few hundred people infected by it over the years, many countries took steps to try to prevent any similar crisis from spreading further.[79] The CDC and other American governmental agencies[80] used the summer lull to take stock of the United States's response to H1N1 flu and attempt to patch any gaps in the public health safety net before flu season started in early autumn.[81] Preparations included planning a second influenza vaccination program in addition to the one for seasonal influenza, and improving coordination between federal, state, and local governments and private health providers.[81] On October 24, 2009, U.S. President Obama declared swine flu a national emergency, giving Secretary of Health and Human Services Kathleen Sebelius authority to grant waivers to requesting hospitals from usual federal requirements.[82]
Vaccines

As of November 19, 2009[update], over 65 million doses of vaccine had been administered in over 16 countries; the vaccine seems safe and effective, producing a strong immune response that should protect against infection.[83] Whereas the trivalent seasonal influenza vaccine neither increases nor decreases the risk of infection with H1N1, the vaccines rushed to combat the new strain are effective against H1N1,[84][85] although they are manufactured similarly. Overall the safety profile of the new H1N1 vaccine is similar to that of the seasonal flu vaccine, and as of November 2009 fewer than a dozen cases of Guillain-Barre syndrome had been reported post-vaccination.[86] Only a few of these are suspected to be actually related to the H1N1 vaccination, and only temporary illness has been observed.[86] This is in strong contrast to the 1976 swine flu outbreak, where mass vaccinations in the United States caused over 500 cases of Guillain-Barre syndrome and led to 25 deaths.[87] Although the vaccines were effective, many industrialized nations were giving away, selling, canceling orders for, and destroying excess doses of the vaccine.[88][89]
There are safety concerns for people who are allergic to eggs because the viruses for the vaccine are grown in chicken-egg-based cultures. People with egg allergies may be able to receive the vaccine, after consultation with their physician, in graded doses in a careful and controlled environment.[90] A vaccine manufactured by Baxter is made without using eggs, but requires two doses three weeks apart to produce immunity.[91]
As of late November, in Canada there had been 24 confirmed cases of anaphylactic shock following vaccination, including one death. The estimated rate is one anaphylactic reaction per 312,000 persons receiving the vaccine, however there had been one batch of vaccine in which six persons suffered anaphylaxis out of 157,000 doses given. Dr. David Butler-Jones, Canada's chief public health officer, stated that even though this was an adjuvanted vaccine, that did not appear to be the cause of this severe allergic reaction in these six patients.[92][93]
In January 2010, Wolfgang Wodarg, a Social Democrat deputy who trained as a doctor and now chairs the health committee at the Council of Europe, claimed major firms organised a "campaign of panic" to put pressure on the World Health Organisation (WHO) to declare a "false pandemic" to sell vaccines. Dr. Wodarg said the WHO's "false pandemic" flu campaign is "one of the greatest medicine scandals of the century." He said that the "false pandemic" campaign began last May in Mexico City, when a hundred or so "normal" reported influenza cases were declared to be the beginning of a threatening new pandemic, although he said there was little scientific evidence for this. Nevertheless he argued that the WHO, "in cooperation with some big pharmaceutical companies and their scientists, re-defined pandemics," removing the statement that "an enormous amount of people have contracted the illness or died" from its existing definition and replacing it by stating simply that there has to be a virus, spreading beyond borders and to which people have no immunity.[94] The WHO has responded by stating that it took its duty to provide independent advice seriously and guarded against interference from outside interests. Announcing a review of its actions, WHO spokeswoman Fadela Chaib stated that, "Criticism is part of an outbreak cycle. We expect and indeed welcome criticism and the chance to discuss it."[95][96] Even so, Wodarg persuaded the Council of Europe to launch an enquiry into 'the influence of the pharmaceutical companies on the global swine flu campaign', and a preliminary report is in preparation.[97] On April 12, 2010, Keiji Fukuda, the WHO's top influenza expert, stated that the system leading to the declaration of a pandemic led to confusion about the flu bug circulating around the world, and he expressed concern that there was a failure to communicate in regard to uncertainties about the new virus, which turned out to be not as deadly as feared. WHO Director-General Margaret Chan has appointed 29 flu experts from outside the organization to conduct a review of WHO's handling of the swine flu pandemic. She has told them, "We want a frank, critical, transparent, credible and independent review of our performance".<refChan M (8 June 2010). "WHO Director-General’s letter to BMJ editors". World Health Organization. http://www.who.int/mediacentre/news/statements/2010/letter_bmj_20100608/en/index.html.</ref>
Infection control
Travel precautions

On May 7, 2009, the WHO stated that containment was not feasible and that countries should focus on mitigating the effect of the virus. It did not recommend closing borders or restricting travel.[98] On April 26, 2009, the Chinese government announced that visitors returning from flu-affected areas who experienced flu-like symptoms within two weeks would be quarantined.[99]
US airlines made no major changes as of the beginning of June 2009, but continued standing practices that included looking for passengers with symptoms of flu, measles, or other infections, and relying on in-flight air filters to ensure that aircraft were sanitized.[100] Masks were not generally provided by airlines and the CDC did not recommend that airline crews wear them.[100] Some non-US airlines, mostly Asian ones, including Singapore Airlines, China Eastern Airlines, China Southern Airlines, Cathay Pacific, and Mexicana Airlines, took measures such as stepping up cabin cleaning, installing state-of-the-art air filters, and allowing in-flight staff to wear face masks.[100]
Schools
US government officials have been especially concerned about schools because the H1N1 flu virus appears to disproportionately affect young and school-age people, between ages six months to 24 years of age.[101] The H1N1 outbreak led to numerous precautionary school closures in some areas. Rather than closing schools, the CDC recommended that students and school workers with flu symptoms should stay home for either seven days total, or until 24 hours after symptoms subside—whichever is longer.[102] The CDC also recommended that colleges should consider suspending fall 2009 classes if the virus began to cause severe illness in a significantly larger share of students than the previous spring. They also urged schools to suspend any rules, including penalizing late papers or missed classes, or requiring a doctor's note, to enforce "self-isolation" and prevent students from venturing out while ill;[103] schools were advised to set aside a room for people developing flu-like symptoms while they waited to go home and that surgical masks be used for ill students or staff and those caring for them.[104]
In California, school districts and universities were on alert and working with health officials to launch education campaigns. Many planned to stockpile medical supplies and discuss worst-case scenarios, including plans to provide lessons and meals for low-income children in case elementary and secondary schools closed.[105] University of California campuses stockpiled supplies, from paper masks and hand sanitizer to food and water.[105] To help prepare for contingencies, University of Maryland School of Medicine professor of pediatrics James C. King Jr. suggested that every county should create an "influenza action team" to be run by the local health department, parents, and school administrators.[106] As of 28 October 2009[update], about 600 Schools in the United States had been temporarily closed, affecting over 126,000 students in 19 states.[107]
Workplace
Fearing a worst-case scenario, the US Department of Health and Human Services (HHS), the Centers for Disease Control and Prevention (CDC), and the US Department of Homeland Security (DHS), developed updated guidance[108] and a video for employers to use as they developed plans to respond to the H1N1 outbreak. The guidance suggested that employers consider and communicate their objectives, which may include reducing transmission among staff, protecting people who are at increased risk of influenza related complications from getting infected with influenza, maintaining business operations, and minimizing adverse effects on other entities in their supply chains.[108]
The CDC estimated that as much as 40% of the workforce might be unable to work at the peak of the pandemic due to the need for many healthy adults to stay home and care for an ill family member,[109] and advised that individuals should have steps in place should a workplace close down or a situation arise that requires working from home.[110] The CDC further advised that persons in the workplace should stay home sick for seven days after getting the flu, or 24 hours after symptoms end, whichever is longer.[102] In the UK, the Health and Safety Executive (HSE) also issued general guidance for employers.[111]
Facial masks
The CDC does not recommend use of face masks or respirators in non-health care settings, such as schools, workplaces, or public places, with a few exceptions: people who are ill with the virus should consider wearing one when around other people, and people who are at risk for severe illness while caring for someone with the flu.[112] There has been some disagreement about the value of wearing facial masks, some experts fearing that masks may give people a false sense of security and should not replace other standard precautions.[113] Masks may benefit people in close contact with infected persons but it was unknown whether they prevent H1N1 flu infection.[113] Yukihiro Nishiyama, professor of virology at Nagoya University's School of Medicine, commented that the masks are "better than nothing, but it's hard to completely block out an airborne virus since it can easily slip through the gaps".[114]
According to mask manufacturer 3M, masks will filter out particles in industrial settings, but "there are no established exposure limits for biological agents such as swine flu virus."[113] However, despite the lack of evidence of effectiveness, the use of such masks is common in Asia.[114][115] Masks are particularly popular in Japan, where cleanliness and hygiene are highly valued and where etiquette obligates those who are sick to wear masks to avoid their spreading disease.[114]
Quarantine
During the height of the fear of a pandemic some countries initiated or threatened to initiate quarantines of foreign visitors suspected of having or being in contact with others who may have been infected. In May, the Chinese government confined 21 US students and three teachers to their hotel rooms.[116] As a result, the US State Department issued a travel alert about China's anti-flu measures and warned travelers about traveling to China if ill.[117] In Hong Kong, an entire hotel was quarantined with 240 guests;[118] Australia ordered a cruise ship with 2,000 passengers to stay at sea because of a swine flu threat.[119] Egyptian Muslims who went on the annual pilgrimage to Mecca risked being quarantined upon their return.[120] Russia and Taiwan said they would quarantine visitors with fevers who come from areas where the flu is present.[121] Japan quarantined 47 airline passengers in a hotel for a week in mid-May,[122] then in mid-June India suggested pre-screening "outbound" passengers from countries thought to have a high rate of infection.[123]
Pigs and food safety
The pandemic virus is a type of swine influenza, derived originally from a strain that lived in pigs and this origin gave rise to the common name of "swine flu". This term is widely used by mass media. The virus has been found in American[124] and Canadian[125] hogs, as well as in hogs in Northern Ireland, Argentina, and Norway.[126] Leading health agencies and the United States Secretary of Agriculture have stressed that eating properly cooked pork or other food products derived from pigs would not cause flu.[127][128] Nevertheless, on April 27, Azerbaijan imposed a ban on the importation of animal husbandry products from America.[129] The Indonesian government also halted the importation of pigs and initiated the examination of 9 million pigs in Indonesia.[130] The Egyptian government ordered the slaughter of all pigs in Egypt on April 29, 2009.[131]
Treatment
A number of methods have been recommended to help ease symptoms, including adequate liquid intake and rest.[132] Over-the-counters pain medications such as acetaminophen and ibuprofen do not kill the virus, however they may be useful to reduce symptoms.[133] Aspirin and other salicylate products should not be used by people under 19 with any flu-type symptoms because of the risk of developing Reye's Syndrome.[134]
If the fever is mild and there are no other complications, fever medication is not recommended.[133] Most people recover without medical attention, although those with pre-existing or underlying medical conditions are more prone to complications and may benefit from further treatments.[40]
People in at-risk groups should be treated with antivirals (oseltamivir or zanamivir) as soon as possible when they first experience flu symptoms. The at risk groups includes pregnant and post partum women, children under two years old, and people with underlying conditions such as respiratory problems.[13] People who are not from the at-risk group who have persistent or rapidly worsening symptoms should also be treated with antivirals. These symptoms include difficulty breathing and a high fever that lasts beyond three days. People who have developed pneumonia should be given both antivirals and antibiotics, as in many severe cases of H1N1-caused illness, bacterial infection develops.[51] Antivirals are most useful if given within 48 hours of the start of symptoms and may improve outcomes in hospitalized patients.[135] In those beyond 48 hours who are moderately or severely ill antiviral may still be beneficial.[11] If oseltamivir (Tamiflu) is unavailable or cannot be used zanamivir (Relenza) is recommended as a substitute.[13][136] Peramivir is an experimental antiviral drug approved for hospitalized patients in cases where the other available methods of treatment are ineffective or unavailable.[137]
To help avoid shortages of these drugs, the CDC recommended oseltamivir treatment primarily for people hospitalized with pandemic flu; people at risk of serious flu complications due to underlying medical conditions; and patients at risk of serious flu complications. The CDC warned that the indiscriminate use of antiviral medications to prevent and treat influenza could ease the way for drug-resistant strains to emerge which would make the fight against the pandemic that much harder. In addition, a British report found that people often failed to complete a full course of the drug or took the medication when not needed.[138]
Side effects
Both medications have known side effects, including lightheadedness, chills, nausea, vomiting, loss of appetite, and trouble breathing. Children were reported to be at increased risk of self-injury and confusion after taking oseltamivir.[132] The WHO warns against buying antiviral medications from online sources, and estimates that half the drugs sold by online pharmacies without a physical address are counterfeit.[139]
Resistance
As of May 22, 2010, CDC has found 67 oseltamivir (Tamiflu)-resistant viruses out of 4,769 tested. This is not totally unexpected as 99.6% of the seasonal H1N1 flu strains tested have developed resistance to oseltamivir.[140] As of May 7, 2010, WHO has received reports of 285 cases of oseltamivir resistance, "with rare onward transmission."[141] Even so, CDC still recommends that persons with flu symptoms who are taking Tamiflu or similar to continue to practice good cough and hand etiquette until their symptoms are over. No circulating flu has yet shown any resistance to zanamivir (Relenza), the other available anti-viral.[11]
Effectiveness of antivirals questioned
On December 8, 2009, the Cochrane Collaboration, which reviews medical evidence, announced in a review published in the British Medical Journal that it had reversed its previous findings that the antiviral drugs oseltamivir (Tamiflu) and zanamivir (Relenza) can ward off pneumonia and other serious conditions linked to influenza. They reported that an analysis of 20 studies showed oseltamivir offered mild benefits for healthy adults if taken within 24 hours of onset of symptoms, but found no clear evidence it prevented lower respiratory tract infections or other complications of influenza.[142][143] Their published finding relates only to its use in healthy adults with influenza; they say nothing about its use in patients judged to be at high risk of complications (pregnant women, children under five, and those with underlying medical conditions), and uncertainty over its role in reducing complications in healthy adults may still leave it as a useful drug for reducing the duration of symptoms. The drugs might eventually be demonstrated to be effective against flu-related complications; in general, the Cochrane Collaboration concluded "Paucity of good data."[143][144]
Some specific results from the British Medical Journal article include, "The efficacy of oral oseltamivir against symptomatic laboratory confirmed influenza was 61% (risk ratio 0.39, 95% confidence interval 0.18 to 0.85) at 75 mg daily ... The remaining evidence suggests oseltamivir did not reduce influenza related lower respiratory tract complications (risk ratio 0.55, 95% confidence interval 0.22 to 1.35)."[143] Notice especially the wide range for this second result.
Epidemiology
| 2009 flu pandemic data | ||
|---|---|---|
| Area | Confirmed deaths |
|
| Worldwide (total) | 14,286 | |
| European Union and EFTA | 2,290 | |
| Other European countries and Central Asia |
457 | |
| Mediterranean and Middle East | 1,450 | |
| Africa | 116 | |
| North America | 3,642 | |
| Central America and Caribbean | 237 | |
| South America | 3,190 | |
| Northeast Asia and South Asia | 2,294 | |
| Southeast Asia | 393 | |
| Australia and Pacific | 217 | |
| Source: ECDC – January 18, 2010[145] | ||
| Further information: Cases and deaths by country | ||
| Note: The ratio of confirmed deaths to total deaths due to the pandemic is unknown. For more information, see "Data reporting and accuracy". |
||
While it is not known precisely where or when the virus originated,[146][147] analyses in scientific journals have suggested that the H1N1 strain responsible for the current outbreak first evolved in September 2008 and circulated amongst humans for several months before being formally recognized and identified as a novel strain of influenza.[146][148]
Mexico
The virus was first reported in two US children in March 2009, but health officials have reported that it apparently infected people as early as January 2009 in Mexico.[149] The outbreak was first detected in Mexico City on March 18, 2009;[150] immediately after the outbreak was officially announced, Mexico notified the US and World Health Organization, and within days of the outbreak Mexico City was "effectively shut down".[151] Some countries canceled flights to Mexico while others halted trade. Calls to close the border to contain the spread were rejected.[151] Mexico already had hundreds of non lethal cases before the outbreak was officially discovered, and was therefore in the midst of a "silent epidemic". As a result, Mexico was reporting only the most serious cases that showed some of the more severe signs different from those of normal flu, possibly leading to a skewed initial estimate of the case fatality rate.[150]
United States
The new strain was first identified by the CDC in two children, neither of whom had been in contact with pigs. The first case, from San Diego County, California, was confirmed from clinical specimens (nasopharyngeal swab) examined by the CDC on April 14, 2009. A second case, from nearby Imperial County, California, was confirmed on April 17. The patient in the first confirmed case had flu symptoms including fever and cough on clinical exam on March 30, and the second on March 28.[152]
The first confirmed H1N1/09 pandemic flu death, which occurred at Texas Children's Hospital in Houston, Texas, was of a toddler from Mexico City visiting family in Brownsville, Texas, before being air-lifted to Houston for treatment.[153]
Data reporting and accuracy
Influenza surveillance information "answers the questions of where, when, and what influenza viruses are circulating. It can be used to determine if influenza activity is increasing or decreasing, but cannot be used to ascertain how many people have become ill with influenza".[154] For example, as of late June 2009 influenza surveillance information showed the US had nearly 28,000 laboratory-confirmed cases including 3,065 hospitalizations and 127 deaths; but mathematical modeling showed an estimated 1 million Americans currently had the 2009 pandemic flu according to Lyn Finelli, a flu surveillance official with the CDC.[155] Estimating deaths from influenza is also a complicated process. In 2005, influenza only appeared on the death certificates of 1,812 people in the US. The average annual US death toll from flu is, however, estimated to be 36,000.[156] The CDC explains[157] that "... influenza is infrequently listed on death certificates of people who die from flu-related complications." and furthermore that "Only counting deaths where influenza was included on a death certificate would be a gross underestimation of influenza's true impact."
With respect to the current H1N1 flu pandemic, influenza surveillance information is available but almost no studies have attempted to estimate the total number of deaths attributable to H1N1 flu. Two studies have been performed by the CDC, however; the most recent estimates that there were 9,820 deaths (range 7,070–13,930) attributable to H1N1 flu from April to November the 14th.[158] During the same period, 1642 deaths were officially confirmed as caused by H1N1 flu.[159][160] The WHO states that total mortality (including deaths unconfirmed or unreported) from H1N1 flu is "unquestionably higher" than its own confirmed death statistics.[161]
The initial outbreak received a week of near-constant media attention. Epidemiologists cautioned that the number of cases reported in the early days of an outbreak can be very inaccurate and deceptive due to several causes, among them selection bias, media bias, and incorrect reporting by governments. Inaccuracies could also be caused by authorities in different countries looking at differing population groups. Furthermore, countries with poor health care systems and older laboratory facilities may take longer to identify or report cases.[162] "...[E]ven in developed countries the [numbers of flu deaths] are uncertain, because medical authorities don't usually verify who actually died of influenza and who died of a flu-like illness."[163] Dr. Joseph S. Bresee (the CDC flu division's epidemiology chief) and Dr. Michael T. Osterholm (director of the Center for Infectious Disease Research) have pointed out that millions of people have had H1N1 flu, usually in a mild form, so the numbers of laboratory-confirmed cases were actually meaningless, and in July 2009 the WHO stopped keeping count of individual cases and focused more on major outbreaks.[164]
History of influenza epidemics
Annual influenza epidemics are estimated to affect 5–15% of the global population. Although most cases are mild, these epidemics still cause severe illness in 3–5 million people and 250,000–500,000 deaths worldwide.[165] On average 41,400 people die each year in the United States based on data collected between 1979 and 2001.[166] In industrialized countries, severe illness and deaths occur mainly in the high-risk populations of infants, the elderly, and chronically ill patients,[165] although the H1N1 flu outbreak (as well as the 1918 Spanish flu) differs in its tendency to affect younger, healthier people.[167]
In addition to these annual epidemics, Influenza A virus strains caused three global pandemics during the 20th century: the Spanish flu in 1918, Asian flu in 1957, and Hong Kong flu in 1968–69. These virus strains had undergone major genetic changes for which the population did not possess significant immunity.[168] Recent genetic analysis has revealed that three-quarters, or six out of the eight genetic segments of the 2009 flu pandemic strain arose from the North American swine flu strains circulating since 1998, when a new strain was first identified on a factory farm in North Carolina, and which was the first-ever reported triple-hybrid flu virus.[169]
The 1918 flu epidemic began with a wave of mild cases in the spring, followed by more deadly waves in the autumn, eventually killing hundreds of thousands in the United States.[170] The great majority of deaths in the 1918 flu pandemic were the result of secondary bacterial pneumonia. The influenza virus damaged the lining of the bronchial tubes and lungs of victims, allowing common bacteria from the nose and throat to infect their lungs. Subsequent pandemics have had many fewer fatalities due to the development of antibiotic medicines that can treat pneumonia.[171]
| Pandemic | Year | Influenza virus type | People infected (approximate) | Estimated deaths worldwide | Case fatality rate |
|---|---|---|---|---|---|
| Spanish flu | 1918–1919 | A/H1N1[172] | 33% (500 million)[173] | 20–100 million[174][175][176] | >2.5%[177] |
| Asian flu | 1956–1958 | A/H2N2[172] | ? | 2 million[176] | <0.1%[177] |
| Hong Kong flu | 1968–1969 | A/H3N2[172] | ? | 1 million[176] | <0.1%[177] |
| Seasonal flu[t 1] | Every year | mainly A/H3N2, A/H1N1, and B | 5–15% (340 million – 1 billion)[178] | 250,000–500,000 per year[165] | <0.1%[179] |
| Swine flu | 2009–2010 | Pandemic H1N1/09 | > 622,482 (lab-confirmed)[180] | 14,286 (lab-confirmed;[t 2] ECDC)[145] ≥8,768 (lab-confirmed;[t 2] WHO)[181] |
0.03%[182] |
- ↑ Not necessarily pandemic, but included for comparison purposes.
- ↑ 2.0 2.1 The ratio of confirmed deaths to total deaths due to pandemic H1N1/09 flu is unknown. For more information, see "Data reporting and accuracy".
The influenza virus has also caused several pandemic threats over the past century, including the pseudo-pandemic of 1947 (thought of as mild because although globally distributed, it caused relatively few deaths),[183] the 1976 swine flu outbreak, and the 1977 Russian flu, all caused by the H1N1 subtype.[168] The world has been at an increased level of alert since the SARS epidemic in Southeast Asia (caused by the SARS coronavirus).[184] The level of preparedness was further increased and sustained with the advent of the H5N1 bird flu outbreaks because of H5N1's high fatality rate, although the strains currently prevalent have limited human-to-human transmission (anthroponotic) capability, or epidemicity.[185]
People who contracted flu before 1957 appeared to have some immunity to H1N1 flu. Dr. Daniel Jernigan of the CDC has stated: "Tests on blood serum from older people showed that they had antibodies that attacked the new virus ... That does not mean that everyone over 52 is immune, since Americans and Mexicans older than that have died of the new flu."[186]
See also
- 2009 flu deaths by region
References
- ↑ International Committee on Taxonomy of Viruses. "The Universal Virus Database, version 4: Influenza A". http://www.ncbi.nlm.nih.gov/ICTVdb/ICTVdB/00.046.0.01.htm.
- ↑ From Caleb Hellerman CNN (2009-06-11). "Swine flu 'not stoppable,' World Health Organization says". CNN.com. http://www.cnn.com/2009/HEALTH/06/11/swine.flu.who/. Retrieved 2010-04-03.
- ↑ Trifonov, Vladimir; Khiabanian, Hossein; Rabadan, Raul (July 9, 2009). "Geographic Dependence, Surveillance, and Origins of the 2009 Influenza A (H1N1) Virus". New England Journal of Medicine 61 (2): 115–119. PMID 19474418. http://content.nejm.org/cgi/content/full/NEJMp0904572. Retrieved 2010-05-14. See Figure 1 in particular.
- ↑ Writing Committee of the WHO Consultation on Clinical Aspects of Pandemic (H1N1) 2009 Influenza (2010). Clinical Aspects of Pandemic 2009 Influenza A (H1N1) Virus Infection. New England Journal of Medicine. pp. 1708–19.
- ↑ 5.0 5.1 "Clinical features of severe cases of pandemic influenza". Geneva, Switzerland: World Health Organization. 2009-10-16. http://www.who.int/csr/disease/swineflu/notes/h1n1_clinical_features_20091016/en/index.html. Retrieved 2009-10-25.
- ↑ Rong-Gong Lin II (November 21, 2009). "When to take a sick child to the ER". Los Angeles Times. http://www.latimes.com/features/health/la-me-flu-guidelines21-2009nov21,0,2717012.story?track=rss. Retrieved 2010-01-04.
- ↑ McNeil, Jr., Donald G. (2009-06-23). "In New Theory, Swine Flu Started in Asia, Not Mexico". The New York Times. http://www.nytimes.com/2009/06/24/health/24flu.html. Retrieved 2009-09-01.
- ↑ Chan, Dr. Margaret (2009-06-11). "World now at the start of 2009 influenza pandemic". World Health Organization. http://www.who.int/mediacentre/news/statements/2009/h1n1_pandemic_phase6_20090611/en/index.html. Retrieved 2009-10-25.
- ↑ 9.0 9.1 "2009 H1N1 Flu ("Swine Flu") and You". Centers for Disease Control and Prevention. 2010-02-10. http://www.cdc.gov/H1N1flu/qa.htm. Retrieved 2010-02-26.
- ↑ Huffstutter, P.J. (2009-12-05). "Don't call it 'swine flu,' farmers implore". Los Angeles Times. http://www.latimes.com/news/nation-and-world/la-na-pigs-flu5-2009dec05,0,6902061.story. Retrieved 2009-12-05.
- ↑ 11.0 11.1 11.2 "Updated Interim Recommendations for the Use of Antiviral Medications in the Treatment and Prevention of Influenza for the 2009–2010 Season". H1N1 Flu. Centers for Disease Control and Prevention. 2009-12-07. http://www.cdc.gov/h1n1flu/recommendations.htm. Retrieved 2009-12-10.
- ↑ Bronze, Michael Stuart (2009-11-13). "H1N1 Influenza (Swine Flu)". eMedicine. Medscape. http://emedicine.medscape.com/article/1673658-overview. Retrieved 2009-12-10.
- ↑ 14.0 14.1 "Pandemic (H1N1) 2009 – update 100". Disease Outbreak News (WHO). 14 May 2010. http://www.who.int/csr/don/2010_05_14/en/index.html. Retrieved 2010-05-14.
- ↑ The Flu Season That Fizzled: Cases of H1N1 Have Dwindled, Seasonal Flu Has Been a No-Show and Doctors Wonder Why, Wall Street Journal, March 2, 2010, Betsy McKay
- ↑ "Global Intensity Map, Week 17 (April 26, 2010-May 2, 2010)". Global Update on 2009 H1N1. WHO. http://gamapserver.who.int/h1n1/qualitative_indicators/atlas.html?indicator=i2&date=Week%2017%20(26-Apr-2010%20:%2002-May-2010). Retrieved 2010-05-14.
- ↑ "Percentage of respiratory specimens that tested positive for influenza: Status as of week 17 (25 April 25 – 01 May 2010)". WHO. http://www.who.int/csr/disease/swineflu/FluTransmissionZones_2010_05_14.png. Retrieved 2010-05-14.
- ↑ "2009 H1N1 Flu: International Situation Update". H1N1 Flu. Centers for Disease Control and Prevention. May 7, 2010. http://www.cdc.gov/h1n1flu/updates/international/. Retrieved 2010-05-14.
- ↑ "WHO declares end of H1N1 pandemic". http://www.pediatricsupersite.com/view.aspx?rid=67459. Retrieved 2010-08-10. "The world is no longer in phase six pandemic alert. We went to the post-pandemic"
- ↑ "H1N1 Still A Pandemic, Says WHO". redOrbit. http://www.redorbit.com/news/health/1893907/h1n1_still_a_pandemic_says_who/. Retrieved 2010-08-10.
- ↑ "Influenza (Seasonal)". World Heath Organization. April 2009. http://www.who.int/mediacentre/factsheets/fs211/en/. Retrieved 2010-02-13.
- ↑ "WHO admits errors in handling flu pandemic: Agency accused of overplaying danger of the virus as it swept the globe". MSNBC.com. 2010-04-12. http://www.msnbc.msn.com/id/36421914/.
- ↑ Lynn, Johnathan (2010-01-12). "WHO to review its handling of H1N1 flu pandemic". Reuters. http://www.reuters.com/article/idUSTRE5BL2ZT20100112.
- ↑ "Swine flu pandemic declared by WHO was 'a waste of money'". London: The Times (UK). 2010-04-22. http://www.timesonline.co.uk/tol/news/world/article7104253.ece.
- ↑ "Transcript of virtual press conference with Dr Keiji Fukuda, Assistant Director-General ad Interim for Health Security and Environment, World Health Organization". World Health Organization. 2009-07-07. http://www.who.int/mediacentre/Pandemic_h1n1_presstranscript_2009_07_07.pdf. Retrieved 2009-10-26.
- ↑ "Interim Novel Influenza A (H1N1) Guidance for Cruise Ships". Centers for Disease Control and Prevention. 2009-08-05. http://www.cdc.gov/h1n1flu/guidance/cruiseships.htm. Retrieved 2009-09-30.
- ↑ Kikon, Chonbenthung S. "SWINE FLU: A pandemic". The Morung Express. http://www.morungexpress.com/analysis/27870.html. Retrieved 2009-09-28.
- ↑ "Renamed swine flu certain to hit Taiwan". The China Post. 2009-04-28. http://www.chinapost.com.tw/health/infectious-diseases/2009/04/29/206057/p2/Renamed-swine.htm. Retrieved 2009-09-26.
- ↑ Bradsher, Keith (28 April 2009). "The naming of swine flu, a curious matter". The New York Times. http://www.nytimes.com/2009/04/29/world/asia/29swine.html. Retrieved 2009-04-29.
- ↑ Pilkington, Ed (28 April 2009). "What's in a name? Governments debate 'swine flu' versus 'Mexican' flu". The Guardian (London). http://www.guardian.co.uk/world/2009/apr/28/mexican-swine-flu-pork-name. Retrieved 29 April 2009.
- ↑ "CDC Briefing on Investigation of Human Cases of H1N1 Flu". Centers for Disease Control and Prevention. 2009-07-24. Archived from the original on 2009-09-08. http://www.webcitation.org/5jeG7e0IC. Retrieved 2009-07-28.
- ↑ "Interim Guidance for 2009 H1N1 Flu (Swine Flu): Taking Care of a Sick Person in Your Home". Centers for Disease Control and Prevention. 2009-08-05. http://www.cdc.gov/h1n1flu/guidance_homecare.htm. Retrieved 2009-11-01.
- ↑ Picard, Andre (2009-11-01). "Reader questions on H1N1 answered". The Globe and Mail (Toronto, Canada). http://www.theglobeandmail.com/life/health/h1n1-swine-flu/updated-reader-questions-on-h1n1-answered/article1329448/. Retrieved 2009-11-02.
- ↑ Hartocollis, Anemona (2009-05-27). "'Underlying conditions' may add to flu worries". The New York Times. http://www.nytimes.com/2009/05/28/health/policy/28flu.html. Retrieved 2009-09-26.
- ↑ Whalen, Jeanne (2009-06-15). "Flu Pandemic Spurs Queries About Vaccine". The Wall Street Journal. Archived from the original on 2009-09-08. http://www.webcitation.org/5jeG3g2RN. Retrieved 2009-08-31.
- ↑ 36.0 36.1 Grady, Denise (2009-09-03). "Report Finds Swine Flu Has Killed 36 Children". The New York Times. http://www.nytimes.com/2009/09/04/health/research/04flu-001.html. Retrieved 2009-09-17.
- ↑ "Surveillance for Pediatric Deaths Associated with 2009 Pandemic Influenza A (H1N1) Virus Infection – United States, April–August 2009". Morbidity and Mortality Weekly Report (Centers for Disease Control and Prevention). 2009-09-04. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5834a1.htm. Retrieved 2009-12-02.
- ↑ 2009–2010 Influenza Season Week 6 ending February 13, 2010, CDC, Fluview: A Weekly Influenza Surveillance Report Prepared by the Influenza Division.
- ↑ "Clinical features of severe cases of pandemic influenza". Pandemic (H1N1) 2009 briefing note 13 (Geneva, Switzerland: World Health Organization). 2009-10-16. http://www.who.int/csr/disease/swineflu/notes/h1n1_clinical_features_20091016/en/index.html. Retrieved 2010-01-17.
- ↑ 40.0 40.1 "What To Do If You Get Sick: 2009 H1N1 and Seasonal Flu". Centers for Disease Control and Prevention. 2009-05-07. http://www.cdc.gov/h1n1flu/sick.htm. Retrieved 2009-11-01.
- ↑ Complications of seasonal and pandemic influenza. PMID 19935413.
- ↑ http://thorax.bmj.com/content/65/7/645.full
- ↑ "Fulminant Myocarditis Associated With Pandemic H1N1 Influenza A Virus in Children". J Am Coll Cardiol 55: 928-9. 2010-02-10. doi:10.1016/j.jacc.2010.01.004. Lay summary. (primary source)
- ↑ "Those With Severe H1N1 At Risk For Pulmonary Emboli, Researchers Find". Sciencedaily.com. 2009-10-18. http://www.sciencedaily.com/releases/2009/10/091014111549.htm. Retrieved 2010-04-03.
- ↑ 45.0 45.1 "Interim Guidance on Specimen Collection, Processing, and Testing for Patients with Suspected Novel Influenza A (H1N1) Virus Infection". CDC.gov. Centers for Disease Control and Prevention. 2009-05-13. http://www.cdc.gov/h1n1flu/specimencollection.htm. Retrieved 2009-11-23.
- ↑ 46.0 46.1 "Influenza Diagnostic Testing During the 2009–2010 Flu Season". H1N1 Flu. Centers for Disease Control and Prevention. 2009-09-29. http://www.cdc.gov/h1n1flu/diagnostic_testing_public_qa.htm. Retrieved 2009-11-23.
- ↑ "Interim Recommendations for Clinical Use of Influenza Diagnostic Tests During the 2009–10 Influenza Season". H1N1 Flu. Centers for Disease Control and Prevention. 2009-09-29. http://www.cdc.gov/h1n1flu/guidance/diagnostic_tests.htm. Retrieved 2009-11-23.
- ↑ "Evaluation of Rapid Influenza Diagnostic Tests for Detection of Novel Influenza A (H1N1) Virus --- United States, 2009". Morbidity and Mortality Weekly Report (Centers for Disease Control and Prevention). 2009-08-07. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5830a2.htm. Retrieved 2009-12-05.
- ↑ "Accuracy of rapid flu tests questioned". HealthFirst. AP (Mid-Michigan, USA: WJRT-TV/DT). 2009-12-01. http://abclocal.go.com/wjrt/story?section=news/health&id=7147447. Retrieved 2009-12-05.
- ↑ "Could Widely Used Rapid Influenza Tests Pose A Dangerous Public Health Risk?". Maywood, Illinois, US: Loyola Medicine. 2009-11-17. http://www.loyolamedicine.org/News/News_Releases/news_release_detail.cfm?var_news_release_id=973441074. Retrieved 2009-12-04.
- ↑ 51.0 51.1 "Transcript of virtual press conference with Gregory Hartl, Spokesperson for H1N1, and Dr Nikki Shindo, Medical Officer, Global Influenza Programme, World Health Organization". World Health Organization. 2009-11-12. http://www.who.int/mediacentre/vpc_transcript_12_november_09_nikki_shindo.pdf. Retrieved 2009-11-18.
- ↑ U.S. Centers for Disease Control (2010-06-22). "New CDC Test to Detect Human Infections with the 2009 H1N1 Influenza Virus Authorized for Use by FDA". Press release. http://www.cdc.gov/media/pressrel/2010/r100622.htm. Retrieved 2010-08-25.
- ↑ Schnirring, Lisa (2009-05-21). "Some immunity to novel H1N1 flu found in seniors". Center for Infectious Disease Research & Policy. http://www.cidrap.umn.edu/cidrap/content/influenza/swineflu/news/may2109serum-jw.html. Retrieved 2009-09-26.
- ↑ "Swine Influenza A (H1N1) Infection in Two Children – Southern California, March–April 2009". Morbidity and Mortality Weekly Report (Centers for Disease Control and Prevention). 21 April 2009. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm58d0421a1.htm. Retrieved 2009-09-26.
- ↑ Chen, Honglin; Wang, Yong; Liu, Wei; Zhang, Jinxia; Dong, Baiqing; Fan, Xiaohui; de Jong, Menno D.; Farrar, Jeremy et al. (November 2009). "Serologic Survey of Pandemic (H1N1) 2009 Virus, Guangxi Province, China". Emerging Infectious Diseases (CDC) 15 (11). doi:10.3201/eid1511.090868.
- ↑ Emma Wilkinson (2009-05-01). "What scientists know about swine flu". BBC News. http://news.bbc.co.uk/2/hi/health/8028371.stm. Retrieved 2009-09-26.
- ↑ Donaldson LJ, Rutter PD, Ellis BM, et al. (2009). "Mortality from pandemic A/H1N1 2009 influenza in England: public health surveillance study". BMJ 339: b5213. doi:10.1136/bmj.b5213. PMID 20007665.
- ↑ Maugh, Thomas H. II (2009-12-11). "Swine flu has hit about one in six Americans, CDC says". Los Angeles Times. http://www.latimes.com/news/nation-and-world/la-sci-swine-flu11-2009dec11,0,6351332.story. Retrieved 2009-12-13.
- ↑ Vijaykrishna D, Poon LL, Zhu HC, Ma SK, Li OT, Cheung CL, Smith GJ, Peiris JS, Guan Y (18 June 2010). "Reassortment of pandemic H1N1/2009 influenza A virus in swine". Science 328 (5985): 1529. doi:10.1126/science.1189132. PMID 20558710.
- ↑ "H1N1 swaps genes with other pig viruses: scientists". The Canadian Press. CTV News. 2010-06-18. http://edmonton.ctv.ca/servlet/an/local/CTVNews/20100618/swine-flu-virus-100618/20100618/?hub=EdmontonHome.
- ↑ Balcan, Duygu; Hu, Hao; Goncalves, Bruno; Bajardi, Paolo; Poletto, Chiara; Ramasco, Jose J.; Paolotti, Daniela; Perra, Nicola et al. (2009-09-14). "Seasonal transmission potential and activity peaks of the new influenza A(H1N1): a Monte Carlo likelihood analysis based on human mobility". BMC Medicine 7 (45): 29. doi:10.1186/1741-7015-7-45. arXiv:0909.2417v1.
- ↑ The New England Journal of Medicine http://content.nejm.org/cgi/content/full/361/27/2619
- ↑ Murray, Louise (2009-11-05). "Can Pets Get Swine Flu?". The New York Times. http://consults.blogs.nytimes.com/2009/11/05/can-pets-get-swine-flu/. Retrieved 2009-11-06.
- ↑ Parker-Pope, Tara (2009-11-05). "The Cat Who Got Swine Flu". The New York Times. http://well.blogs.nytimes.com/2009/11/05/the-cat-who-got-swine-flu/. Retrieved 2009-11-06.
- ↑ "Pet dog recovers from H1N1". CBCNews. 2009-12-22. http://www.cbc.ca/health/story/2009/12/22/h1n1-us-dog-who.html.
- ↑ "2009 Pandemic H1N1 Influenza Presumptive and Confirmed Results". US Department of Agriculture. 2009-12-04. http://www.usda.gov/documents/FINAL_RESULTS_2009_PANDEMIC_H1N1_INFLUENZA_CHT.pdf. Retrieved 2009-12-04.
- ↑ "Use of Influenza A (H1N1) 2009 Monovalent Vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009". Morbidity and Mortality Weekly Report. Centers for Disease Control and Prevention. 2009-11-28. http://www.cdc.gov/mmWR/PDF/rr/rr5810.pdf. Retrieved 2009-11-02.
- ↑ 68.0 68.1 "Swine flu latest from the NHS". NHS Choices. NHS Knowledge Service (NHS). 2009-09-25. http://www.nhs.uk/news/2009/04april/pages/swineflulatest.aspx. Retrieved 2009-09-28.
- ↑ 69.0 69.1 McNeil, Jr., Donald G. (2009-09-10). "One Vaccine Shot Seen as Protective for Swine Flu". The New York Times. http://www.nytimes.com/2009/09/11/health/11vaccine.html?_r=1. Retrieved 2009-09-17.
- ↑ "Experts advise WHO on pandemic vaccine policies and strategies". World Health Organization. 30 October 2009. http://www.who.int/csr/disease/swineflu/notes/briefing_20091030/en/index.html. Retrieved 2009-11-02.
- ↑ Fox, Maggie (21 September 2009). "Young children need 2 doses of H1N1 vaccine – US". Reuters. http://www.reuters.com/article/healthNews/idUSTRE58H4EP20090921. Retrieved 2009-09-22.
- ↑ Dr. Anthony Fauci, Director, National Institute of Allergy and Infectious Diseases, NIH (21 September 2009). Update on NIAID Clinical Trials of 2009 H1N1 Influenza Vaccines in Children. NIAID. http://services.choruscall.com/links/nih090921.html. Retrieved 2009-09-22. "Children from six months to nine years old may require two vaccinations [closed caption, app. five minutes into presentation]"
- ↑ Fox, Maggie (2009-07-24). "First defense against swine flu – seasonal vaccine". Reuters. http://www.reuters.com/article/GCA-SwineFlu/idUSTRE56N3PQ20090724. Retrieved 2009-09-17.
- ↑ McKay, Betsy (2009-07-18). "New Push in H1N1 Flu Fight Set for Start of School". The Wall Street Journal. Archived from the original on 2009-09-08. http://www.webcitation.org/5jeG4aNty. Retrieved 2009-08-31.
- ↑ "Vaccine Selection for the 2010–2011 Influenza Season". U.S. Centers for Disease Control. http://www.cdc.gov/flu/about/qa/1011_vac_selection.htm. Retrieved 2010-08-25.
- ↑ Nasaw, Daniel (April 27, 2009). "Europeans urged to avoid Mexico and US as swine flu death toll exceeds 100". Guardian (London). http://www.guardian.co.uk/world/2009/apr/27/swine-flu-mexico. Retrieved 2009-04-27.
- ↑ AFP (2009-05-06). "H1N1 virus genome: 'This is a world first'". Independent. Archived from the original on 2009-09-08. http://www.webcitation.org/5jeF2dg9n. Retrieved 2009-08-31.
- ↑ "National Pandemic Flu Service". NHS, NHS Scotland, NHS Wales, DHSSPS. https://www.pandemicflu.direct.gov.uk/. Retrieved 2009-10-04.
- ↑ Hines, Lora (2009-06-07). "Health officials evaluate response to swine flu". Riverside Press-Enterprise. http://www.pe.com/localnews/rivcounty/stories/PE_News_Local_S_swineflu08.4301c9d.html. Retrieved 2009-09-17.
- ↑ Stein, Rob (2009-08-10). "Preparing for Swine Flu's Return". The Washington Post. http://www.washingtonpost.com/wp-dyn/content/article/2009/08/09/AR2009080902447.html. Retrieved 2009-10-04.
- ↑ 81.0 81.1 Steenhuysen, Julie (2009-06-04). "As swine flu wanes, U.S. preparing for second wave". Reuters. http://www.reuters.com/article/domesticNews/idUSN0423008520090604?sp=true. Retrieved 2009-09-17.
- ↑ Shear, Michael D.; Stein, Rob (2009-10-24). "President Obama declares H1N1 flu a national emergency". The Washington Post. http://www.washingtonpost.com/wp-dyn/content/article/2009/10/24/AR2009102401061.html. Retrieved 2009-10-25.
- ↑ Greenberg, Michael E.; Michael H. Lai, Gunter F. Hartel, Christine H. Wichems, Charmaine Gittleson, Jillian Bennet, Gail Dawson, Wilson Hu, Connie Leggio, Diane Washington, Russell L. Basser (2009). "Response after One Dose of a Monovalent Influenza A (H1N1) 2009 Vaccine – Preliminary Report". N Engl J Med 361: NEJMoa0907413. doi:10.1056/NEJMoa0907413. http://content.nejm.org/cgi/content/abstract/NEJMoa0907413v1. Lay summary.
- ↑ Centers for Disease Control and Prevention (November 2009). "Effectiveness of 2008–09 trivalent influenza vaccine against 2009 pandemic influenza A (H1N1) – United States, May–June 2009". MMWR 58 (44): 1241–5. PMID 19910912. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5844a5.htm.
- ↑ Hancock K, Veguilla V, Lu X, et al. (November 2009). "Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus". N. Engl. J. Med. 361 (20): 1945–52. doi:10.1056/NEJMoa0906453. PMID 19745214.
- ↑ 86.0 86.1 "Transcript of virtual press conference with Dr Marie-Paule Kieny, Director, Initiative for Vaccine Research World Health Organization". World Health Organization. 2009-11-19. p. 5. http://www.who.int/mediacentre/vpc_transcript_19_november_09_kieny.pdf. Retrieved 2009-11-22.
- ↑ Roan, Shari (2009-04-27). "Swine flu 'debacle' of 1976 is recalled". Los Angeles Times. http://articles.latimes.com/2009/apr/27/science/sci-swine-history27?pg=2. Retrieved 2009-11-19.
- ↑ Britain Slashed Swine Flu Vaccine Order, The Independent, Alistair Dawber.
- ↑ H1N1 Vaccines Lie Unused, Might be Disposed, Top News, Jason Ramsey, 04/08/2010.
- ↑ "Have Egg Allergy? You May Still Be Candidate for Flu Vaccines, Says Allergist". Infection Control Today (Virgo Publishing). 2009-11-18. http://www.infectioncontroltoday.com/hotnews/egg-allergy-and-flu-vaccines.html. Retrieved 2009-11-22.
- ↑ "GPs to receive swine flu vaccines". BBC News (BBC). 2009-10-26. http://news.bbc.co.uk/2/hi/health/8325363.stm. Retrieved 2009-11-22.
- ↑ Canada Probes H1N1 Vaccine Anaphylaxis Spike, Michael Smith, North American Correspondent, MedPage Today, Nov. 30, 2009.
- ↑ "Transcript of virtual press conference with Kristen Kelleher, Communications Officer for pandemic (H1N1) 2009, and Dr Keiji Fukuda, Special Adviser to the Director-General on Pandemic Influenza". World Health Organization. 2009-11-26. http://www.who.int/mediacentre/vpc_transcript_26_november_09_fukuda.pdf. Retrieved 2009-12-01.
- ↑ Pollard, Chris (2010-01-13). "Swine flu 'a false pandemic' to sell vaccines, expert says". News.com. http://www.news.com.au/world/swine-flu/story-e6frfkyi-1225818388508. Retrieved 2010-01-13.
- ↑ Stephanie Nebehay WHO to review its handling of H1N1 flu pandemic Reuters Tue Jan 12
- ↑ Bloomberg http://www.bloomberg.com/apps/news?pid=20601091&sid=ahj0H_RH8U68
- ↑ http://assembly.coe.int/CommitteeDocs/2010/AS_Inf_2010_E.pdf
- ↑ "WHO – Influenza A(H1N1) – Travel". World Health Organization. 2009-05-07. http://www.who.int/csr/disease/swineflu/frequently_asked_questions/travel/en/index.html. Retrieved 2009-06-17.
- ↑ "FACTBOX-Asia moves to ward off new flu virus". Reuters. 2009-02-09. http://www.reuters.com/article/europeCrisis/idUST344175. Retrieved 2009-04-26.
- ↑ 100.0 100.1 100.2 Jacobs, Karen (2009-06-03). "Global airlines move to reduce infection risks". Reuters. http://www.reuters.com/article/asiaCrisis/idUSN27536654. Retrieved 2009-09-17.
- ↑ "New CDC H1N1 Guidance for Colleges, Universities, and Institutions of Higher Education". Business Wire. 2009-08-20. http://www.businesswire.com/portal/site/google/?ndmViewId=news_view&newsId=20090820006035&newsLang=en. Retrieved 2009-09-17.
- ↑ 102.0 102.1 George, Cindy (2009-08-01). "Schools revamp swine flu plans for fall". Houston Chronicle. Archived from the original on 2009-09-08. http://www.webcitation.org/5jeG8PPyY. Retrieved 2009-08-31.
- ↑ de Vise, Daniel (2009-08-20). "Colleges Warned About Fall Flu Outbreaks on Campus". The Washington Post. http://www.washingtonpost.com/wp-dyn/content/article/2009/08/20/AR2009082003449.html?hpid=moreheadlines. Retrieved 2009-09-17.
- ↑ "Get Smart About Swine Flu for Back-to-School". Atlanta Journal-Constitution. 2009-08-14. Archived from the original on 2009-09-08. http://www.webcitation.org/5jeG9XwyO. Retrieved 2009-08-31.
- ↑ 105.0 105.1 Mehta, Seema; Nicole Santa Cruz (2009-07-27). "Swine flu goes to camp. Will it go to school next?". Los Angeles Times. http://www.latimes.com/features/health/la-me-camp-flu27-2009jul27,0,5762666.story. Retrieved 2009-09-17.
- ↑ James C. Jr., King (2009-08-01). "The ABC's of H1N1". The New York Times. http://www.nytimes.com/2009/08/02/opinion/02king.html. Retrieved 2009-09-17.
- ↑ "H1N1 Closes Hundreds of Schools Across the U.S.". Fox News. 2009-10-28. http://www.foxnews.com/story/0,2933,570129,00.html. Retrieved 2009-10-28.
- ↑ 108.0 108.1 "Business Planning". Flu.gov. U.S. Department of Health & Human Services. http://www.flu.gov/plan/workplaceplanning/guidance.html. Retrieved 2009-09-17.
- ↑ Maugh II, Thomas H. (2009-07-25). "Swine flu could kill hundreds of thousands in U.S. if vaccine fails, CDC says". Los Angeles Times. http://www.latimes.com/features/health/la-sci-swine-flu25-2009jul25,0,3387335.story. Retrieved 2009-09-17.
- ↑ Fiore, Marrecca (2009-07-17). "Swine Flu: Why You Should Still Be Worried". Fox News. Archived from the original on 2009-09-08. http://www.webcitation.org/5jeG55WpO. Retrieved 2009-08-31.
- ↑ "Swine flu – HSE News Announcement". Health and Safety Executive. 2009-06-18. http://www.hse.gov.uk/news/2009/swineflu.htm. Retrieved 2009-08-31.
- ↑ Roan, Shari (2009-09-27). "Masks may help prevent flu, but aren't advised". Chicago Tribune. http://www.chicagotribune.com/entertainment/chi-tc-health-flu-masks-0923sep27,0,7442578.story. Retrieved 2009-09-28.
- ↑ 113.0 113.1 113.2 Roan, Shari; Rong-Gong Lin II (2009-04-30). "Face masks aren't a sure bet against swine flu". Los Angeles Times. http://www.latimes.com/features/health/la-sci-swine-mask30-2009apr30,0,7635596.story. Retrieved 2009-09-17.
- ↑ 114.0 114.1 114.2 "Face masks part of Japan fashion chic for decades". Bangkok Post. AFP (The Post Publishing). 2009-04-05. http://www.bangkokpost.com/news/asia/142274/face-masks-part-of-japan-fashion-chic-for-decades. Retrieved 2009-10-28.
- ↑ Wohl, Jessica (2009-10-20). "Flu-Related Products May Lift U.S. Makers' Profits". ABC News.com (ABC News). http://abcnews.go.com/Business/wireStory?id=8870908. Retrieved 2009-10-28.
- ↑ "China quarantines U.S. school group over flu concerns". CNN. 2009-05-28. http://www.cnn.com/2009/HEALTH/05/28/us.china.swine.flu/. Retrieved 2009-09-26.
- ↑ Kralev, Nicholas (2009-06-29). "KRALEV: U.S. warns travelers of China's flu rules". The Washington Times. http://washingtontimes.com/news/2009/jun/29/kralev-us-warns-travelers-of-chinas-flu-rules/. Retrieved 2009-09-26.
- ↑ DPA (2009-05-03). "Tensions escalate in Hong Kong's swine-flu hotel". Taipei Times. http://www.taipeitimes.com/News/world/archives/2009/05/03/2003442656. Retrieved 2009-09-26.
- ↑ "Ship passengers cruisy in swine flu quarantine". ABC News (Australian Broadcasting Corporation). 2009-05-28. http://www.abc.net.au/news/stories/2009/05/28/2583515.htm?section=justin. Retrieved 2009-09-17.
- ↑ El Deeb, Sarah (2009-05-20). "Egypt Warns of Post-Hajj Swine Flu Quarantine". Cairo: ABC News. http://abcnews.go.com/International/wireStory?id=7634743. Retrieved 2009-09-27.
- ↑ Woolls, Daniel (2009-04-28). "Swine flu cases in Europe; worldwide travel shaken". Taiwan News. AP. http://www.etaiwannews.com/etn/news_content.php?id=932378&lang=eng_news&cate_img=316.jpg&cate_rss=news_Health. Retrieved 2009-09-26.
- ↑ "Japan Swine Flu Quarantine Ends for Air Passengers". New Tang Dynasty Television. 2009-05-17. http://www.youtube.com/watch?v=P6fEMGHoQPE. Retrieved 2009-09-26.
- ↑ MIL/Sify.com (2009-06-16). "India wants US to screen passengers for swine flu". International Reporter. http://www.internationalreporter.com/News-4898/india-wants-us-to-screen-passengers-for-swine-flu.html. Retrieved 2009-09-28.
- ↑ Jordan, Dave (2009-10-20). "Minnesota Pig Tests Positive for H1N1". KOTV. http://www.newson6.com/Global/story.asp?S=11341148. Retrieved 2009-12-10.
- ↑ Evans, Brian (2009-05-05). "News Conference with Minister of Health and Chief Public Health Officer". Public Health Agency of Canada. http://www.phac-aspc.gc.ca/alert-alerte/h1n1/conferences/nc-2009-05-02-eng.php. Retrieved 2009-12-10.
- ↑ "Human swine flu in pigs". Effect Measure. 2009-10-20. http://scienceblogs.com/effectmeasure/2009/10/human_swine_flu_in_pigs.php. Retrieved 2009-12-10.
- ↑ World Health Organization (2009-05-07). "Joint FAO/WHO/OIE Statement on influenza A(H1N1) and the safety of pork". Press release. http://www.who.int/mediacentre/news/statements/2009/h1n1_20090430/en/index.html. Retrieved 2009-09-26.
- ↑ Desmon, Stephanie (2009-08-21). "Pork industry rues swine flu". The Baltimore Sun 172 (233): pp. 1, 16. Archived from the original on 2009-09-08. http://www.webcitation.org/5jeGAPswM. Retrieved 2009-09-01.
- ↑ "Prevention against "swine flu" stabile in Azerbaijan: minister". Trend News Agency. 2009-04-28. http://news-en.trend.az/society/health/1462401.html. Retrieved 2009-04-28.
- ↑ "Cegah flu babi, pemerintah gelar rapat koordinasi". Kompas newspaper. 2009-04-27. http://nasional.kompas.com/read/xml/2009/04/27/11120229/cegah.flu.babi.pemerintah.gelar.rapat.koordinasi.
- ↑ "Egypt orders pig cull". ABC News. AFP (Australian Broadcasting Corporation). 2009-04-30. http://www.abc.net.au/news/stories/2009/04/30/2556545.htm?section=justin%20Egypt%20orders%20pig%20cull. Retrieved 2009-09-27.
- ↑ 132.0 132.1 Mayo Clinic Staff. "Influenza (flu) treatments and drugs". Diseases and Conditions. Mayo Clinic. Archived from the original on 2009-09-08. http://www.webcitation.org/5jeG5VTNF. Retrieved 2009-05-20.
- ↑ 133.0 133.1 "Fever". Medline Plus Medical Encyclopedia. U.S. National Library of Medicine. http://www.nlm.nih.gov/medlineplus/ency/article/003090.htm. Retrieved 2009-05-20.
- ↑ "Aspirin / Salicylates and Reyes Syndrome". National Reye's Syndrome Foundation. http://www.reyessyndrome.org/aspirin.html.
- ↑ Jain S, Kamimoto L, Bramley AM, et al. (November 2009). "Hospitalized patients with 2009 H1N1 influenza in the United States, April–June 2009". N. Engl. J. Med. 361 (20): 1935–44. doi:10.1056/NEJMoa0906695. PMID 19815859.
- ↑ + 24-Oct-2009+PRN20091024 "Emergency Use Authorization Granted For BioCryst's Peramivir". PRNewswire-FirstCall. Reuters. 2009-10-23. http://www.reuters.com/article/pressRelease/idUS10540 + 24-Oct-2009+PRN20091024. Retrieved 2009-11-18.
- ↑ Cheng, Maria (2009-08-21). "WHO: Healthy people who get swine flu don't need Tamiflu; drug for young, old, pregnant". Washington Examiner. http://www.washingtonexaminer.com/economy/ap/53932902.html. Retrieved 2009-09-17.
- ↑ BMJ Group (2009-05-08). "Warning against buying flu drugs online". The Guardian (London). http://www.guardian.co.uk/lifeandstyle/besttreatments/2009/may/08/warning-against-buying-flu-drugs-online. Retrieved 2009-09-26.
- ↑ "2008–2009 Influenza Season Week 39 ending October 3, 2009". Centers for Disease Control and Prevention (CDC). 2009-10-09. http://www.cdc.gov/flu/weekly/weeklyarchives2008-2009/weekly39.htm. Retrieved 2009-11-20.
- ↑ Pandemic (H1N1) 2009 – update 99, WHO, Weekly virological surveillance update, 9 April 2010. "All of these viruses showed the H275Y substitution and all remain sensitive to zanamivir."
- ↑ Cortez, Michelle Fay (2009-12-09). "Roche's Tamiflu Not Proven to Cut Flu Complications, Study Says". Bloomberg.com (Bloomberg L.P). http://www.bloomberg.com/apps/news?pid=20601100&sid=a9acc7qIJFdU. Retrieved 2009-12-11.
- ↑ 143.0 143.1 143.2 Jefferson, Tom; Jones, Mark; Doshi, Peter; Del Mar, Chris (2009-12-08). "Neuraminidase inhibitors for preventing and treating influenza in healthy adults: systematic review and meta-analysis". BMJ (BMJ Publishing Group Ltd.) 339: b5106. doi:10.1136/bmj.b5106. PMID 19995812. PMC 2790574. http://www.bmj.com/cgi/content/abstract/339/dec07_2/b5106?ijKey=b9e57312b08cf03c1923f6a254048e5d5a08e60d&keytype2=tf_ipsecsha. Retrieved 2009-12-11.
- ↑ Godlee, Fiona (2009-12-10). "We want raw data, now". BMJ (BMJ Publishing Group Ltd.) 339: b5405. doi:10.1136/bmj.b5405. http://www.bmj.com/cgi/content/full/339/dec10_2/b5405.
- ↑ 146.0 146.1 Check, Hayden Erica (2009-05-05). "The turbulent history of the A(H1N1) virus" (fee required). Nature 459: 14. doi:10.1038/459014a. ISSN 1744-7933. http://www.nature.com/news/2009/090505/full/459014a/box/1.html. Retrieved 2009-05-07.
- ↑ Larry Pope (flv). Smithfield Foods CEO on flu virus. [Television production]. MSNBC. http://www.msnbc.msn.com/id/21134540/vp/30523283#30523283. Retrieved 2009-09-16.
- ↑ Cohen Jon, Enserink Martin (2009-05-01). "As Swine Flu Circles Globe, Scientists Grapple With Basic Questions" (fee required). Science 324 (5927): 572–3. doi:10.1126/science.324_572. http://www.sciencemag.org/cgi/content/full/324/5927/572. Retrieved 2009-09-26.
- ↑ Fox, Maggie (2009-06-11). "New flu has been around for years in pigs – study". Reuters. http://www.reuters.com/article/middleeastCrisis/idUSN11399103. Retrieved 2009-09-17.
- ↑ 150.0 150.1 McNeil Jr., Donald G. (2009-04-26). "Flu Outbreak Raises a Set of Questions". The New York Times. http://www.nytimes.com/2009/04/27/health/27questions.html. Retrieved 2009-09-17.
- ↑ 151.0 151.1 "Study: Mexico Has Thousands More Swine Flu Cases". FOXNews.com. Associated Press (FOX News Network). 2009-05-12. http://www.foxnews.com/story/0,2933,519902,00.html?sPage=fnc/health/infectious. Retrieved 2009-09-17.
- ↑ "Swine Influenza A (H1N1) Infection in Two Children --- Southern California, March–April 2009". Morbidity and Mortality Weekly Report. Centers for Disease Control and Prevention. 2009-04-24. pp. 400–402. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5815a5.htm. Retrieved 2009-10-04.
- ↑ "Swine flu victim dies in Houston". AP. Houston, Texas: KTRK-TV/DT. 2009-04-29. http://abclocal.go.com/ktrk/story?section=news/health&id=6786416&rss=rss-ktrk-article-6786416. Retrieved 2009-11-18.
- ↑ "Flu Activity & Surveillance". Seasonal Influenza (Flu). Centers for Disease Control and Prevention. 2009-11-30. http://www.cdc.gov/flu/weekly/fluactivity.htm. Retrieved 2009-12-02.
- ↑ Stobbe, Mike (2009-06-25). "US Swine Flu Cases May Have Hit 1 Million". The Huffington Post. AP. http://www.huffingtonpost.com/2009/06/25/us-swine-flu-cases-may-ha_n_221240.html. Retrieved 2009-12-02.
- ↑ Ellenberg, Jordan (2009-07-04). "Influenza Body Count". Slate. http://www.slate.com/id/2218367/. Retrieved 2009-12-02.
- ↑ "Questions and Answers Regarding Estimating Deaths from Influenza in the United States". Seasonal Influenza (Flu). Centers for Disease Control and Prevention. 2009-09-04. http://www.cdc.gov/flu/about/disease/us_flu-related_deaths.htm. Retrieved 2 December 2009.
- ↑ "CDC Estimates of 2009 H1N1 Influenza Cases, Hospitalizations and Deaths in the United States, April – December 12, 2009". H1N1 Flu. CDC. 2010-01-15. http://www.cdc.gov/h1n1flu/estimates_2009_h1n1.htm. Retrieved 2010-01-17.
- ↑ "CDC week 45". Centers for Disease Control and Prevention. 2009-11-14. http://www.cdc.gov/flu/weekly/weeklyarchives2009-2010/weekly45.htm. Retrieved 14 December 2009.
- ↑ "CDC week 34". Centers for Disease Control and Prevention. 2009-08-30. http://www.cdc.gov/flu/weekly/weeklyarchives2008-2009/weekly34.htm. Retrieved 14 December 2009.
- ↑ Gale, Jason (2009-12-04). "Clinical features of severe cases of pandemic influenza". Bloomberg.com (Bloomberg). http://www.bloomberg.com/apps/news?pid=20601124&sid=akODnI6cicxw. Retrieved 2009-12-04.
- ↑ "GLOBAL: No A (H1N1) cases – reality or poor lab facilities?". Reuters. 2009-05-08. http://www.alertnet.org/thenews/newsdesk/IRIN/2687d9562c28b487c30aca4e889c5e93.htm. Retrieved 2009-05-09.
- ↑ Sandman, Peter M.; Lanard, Jody (2005). "Bird Flu: Communicating the Risk". Perspectives in Health Magazine 10 (2). http://www.paho.org/English/DD/PIN/Number22_article1a.htm.
- ↑ "Pandemic (H1N1) 2009 briefing note 3" (in 2009-07-16). World Health Organization. http://www.who.int/csr/disease/swineflu/notes/h1n1_surveillance_20090710/en/index.html. Retrieved 2009-07-17.
- ↑ 165.0 165.1 165.2 "Influenza: Fact sheet". World Health Organization. March 2003. http://www.who.int/mediacentre/factsheets/2003/fs211/en/. Retrieved 2009-05-07.
- ↑ Dushoff, J.; Plotkin, J. B.; Viboud, C.; Earn, D. J. D.; Simonsen, L. (2006). "Mortality due to Influenza in the United States – An Annualized Regression Approach Using Multiple-Cause Mortality Data". American Journal of Epidemiology 163: 181–7. doi:10.1093/aje/kwj024. http://aje.oxfordjournals.org/cgi/content/full/163/2/181. Retrieved 2009-10-29. "The regression model attributes an annual average of 41,400 (95% confidence interval: 27,100, 55,700) deaths to influenza over the period 1979–2001.".
- ↑ Kaplan, Karen (2009-09-18). "Swine flu's tendency to strike the young is causing confusion". Los Angeles Times. http://bulletin.aarp.org/yourhealth/diseases/articles/swine_flus_tendency_to_strike_the_young_is_causing_confusion.html. Retrieved 2009-09-18.
- ↑ 168.0 168.1 Kilbourne, ED; Zhang, Yan B.; Lin, Mei-Chen (January 2006). "Influenza pandemics of the 20th century.". Emerging Infectious Diseases (Centers for Disease Control and Prevention) 12 (1): 299. doi:10.1177/1461444804041438. http://www.cdc.gov/ncidod/EID/vol12no01/05-1254.htm. Retrieved 2009-09-26.
- ↑ Greger, Dr. Michael (2009-08-26). "CDC Confirms Ties to Virus First Discovered in U.S. Pig Factories". The Humane Society of the United States. Archived from the original on 2009-09-26. http://www.webcitation.org/5k5hTrO4Y. Retrieved 2009-09-21.
- ↑ Sternberg, Steve (2009-05-26). "CDC expert says flu outbreak is dying down – for now". USA Today. http://www.usatoday.com/news/health/2009-05-26-swine-flu-decrease_N.htm. Retrieved 2009-09-17.
- ↑ David M. Morens, Jeffery K. Taubenberger, and Anthony S. Fauci (2008-10-01). "Predominant Role of Bacterial Pneumonia as a Cause of Death in Pandemic Influenza: Implications for Pandemic Influenza Preparedness". The Journal of Infectious Diseases 198 (7): 962–970. doi:10.1086/591708. http://www.journals.uchicago.edu/doi/full/10.1086/591708. Retrieved 2009-11-01.
- ↑ 172.0 172.1 172.2 Hsieh, Yu-Chia; et al. (January 2006). "Influenza pandemics: past present and future" (PDF). Journal of the Formosan Medical Association 105 (1): 1–6. doi:10.1016/S0929-6646(09)60102-9. PMID 16440064. http://ajws.elsevier.com/ajws_archive/200611051A1086.pdf. Retrieved 2009-09-26.
- ↑ Taubenberger, Jeffery K.; Morens, David M. (January 2006). "1918 Influenza: the Mother of All Pandemics". Emerging Infectious Diseases (Centers for Disease Control and Prevention). http://www.cdc.gov/ncidod/eid/vol12no01/05-0979.htm. Retrieved 2009-05-09.
- ↑ Knobler S, Mack A, Mahmoud A, Lemon S, ed. "1: The Story of Influenza". The Threat of Pandemic Influenza: Are We Ready? Workshop Summary (2005). Washington, D.C.: The National Academies Press. pp. 60–61. http://books.nap.edu/openbook.php?record_id=11150&page=58. Retrieved 2009-09-26.
- ↑ Patterson, KD; Pyle GF (Spring 1991). "The geography and mortality of the 1918 influenza pandemic". Bull Hist Med. 65 (1): 4–21. PMID 2021692.
- ↑ 176.0 176.1 176.2 "Ten things you need to know about pandemic influenza". World Health Organization. 2005-10-14. http://www.who.int/csr/disease/influenza/pandemic10things/en/index.html. Retrieved 2009-09-26.
- ↑ 177.0 177.1 177.2 Taubenberger, JK; Morens, DM (January 2006). "1918 influenza: the mother of all pandemics". Emerging Infectious Diseases (Centers for Disease Control and Prevention) 12 (1). http://www.cdc.gov/ncidod/eid/vol12no01/05-0979.htm. Retrieved 2009-09-26.
- ↑ "WHO Europe – Influenza". World Health Organization. June 2009. http://www.euro.who.int/influenza. Retrieved 2009-06-12.
- ↑ Reuters (2009-09-17). "H1N1 fatality rates comparable to seasonal flu". The Malaysian Insider (Washington, D.C., USA). http://www.themalaysianinsider.com/index.php/world/37983-h1n1-fatality-rates-comparable-to-seasonal-flu. Retrieved 2009-09-26.
- ↑ "Pandemic (H1N1) 2009 – update 76". Global Alert and Response (GAR). World Health Organization. 2009-11-27. http://www.who.int/csr/don/2009_11_27a/en/index.html. Retrieved 2009-12-11.
- ↑ "Pandemic (H1N1) 2009 – update 77". Global Alert and Response (GAR). World Health Organization. 2009-12-04. http://www.who.int/csr/don/2009_12_04/en/index.html. Retrieved 2009-12-11.
- ↑ Triggle, Nick (2009-12-10). "Swine flu less lethal than feared". BBC News (BBC). http://news.bbc.co.uk/2/hi/health/8406723.stm. Retrieved 2009-12-10.
- ↑ Kilbourne, Edwin D. (January 2006). "Influenza Pandemics of the 20th Century". Emerging Infectious Diseases (CDC) 12 (1): 9–14. PMID 16494710. http://www.cdc.gov/ncidod/eid/vol12no01/pdfs/05-1254.pdf. Retrieved 2009-12-10.
- ↑ Brown, David (2009-04-29). "System set up after SARS epidemic was slow to alert global authorities". The Washington Post. http://www.washingtonpost.com/wp-dyn/content/article/2009/04/29/AR2009042904911.html. Retrieved 2009-09-26.
- ↑ Beigel JH, Farrar J, Han AM, Hayden FG, Hyer R, de Jong MD, Lochindarat S, Nguyen TK, Nguyen TH, Tran TH, Nicoll A, Touch S, Yuen KY; Writing Committee of the World Health Organization (WHO) Consultation on Human Influenza A/H5 (September 29, 2005). "Avian influenza A (H5N1) infection in humans". The New England Journal of Medicine 353 (13): 1374–85. doi:10.1056/NEJMra052211. PMID 16192482. http://content.nejm.org/cgi/content/full/353/13/1374.
- ↑ McNeil Jr., Donald G. (2009-05-20). "U.S. Says Older People Appear Safer From New Flu Strain". The New York Times. http://www.nytimes.com/2009/05/21/health/21swineflu.html. Retrieved 2009-09-26.
Further reading
- Cannell, JJ; Zasloff, M; Garland, CF; Scragg, R; Giovannucci, E (2008-02-25). "On the epidemiology of influenza". Virology Journal 5 (29): 29. doi:10.1186/1743-422X-5-29. PMID 18298852. PMC 2279112. http://www.virologyj.com/content/5/1/29.
- Smith, GJ; Vijaykrishna, D; Bahl, J; Lycett, SJ; Worobey, M; Pybus, OG; Ma, SK; Cheung, CL et al.; et al. (2009-06-25). "Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic". Nature 459 (459): 1122–1125. doi:10.1038/nature08182. PMID 19516283.
- Soundararajan, V; Tharakaraman, K; Raman, R; Raguram, S; Shriver, Z; Sasisekharan, V; Sasisekharan, R; et al. (June 2009). "Extrapolating from sequence—the 2009 H1N1 'swine' influenza virus". Nature Biotechnology 27 (6): 510–513. doi:10.1038/nbt0609-510. PMID 19513050. http://www.nature.com/nbt/journal/v27/n6/pdf/nbt0609-510.pdf.
- "Introduction and Transmission of 2009 Pandemic Influenza A (H1N1) Virus – Kenya, June–July 2009". Morbidity and Mortality Weekly Report. Centers for Disease Control and Prevention (CDC). 2009-10-23. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5841a1.htm. Retrieved 2009-10-23.
External links
| Wikinews has news related to:
Swine flu
|
- Influenza: H1N1 at the Open Directory Project
- Pandemic (H1N1) 2009 at the World Health Organization
- International Society for Infectious Diseases PROMED-mail news updates
- H1N1 Flu Resource Centre of The Lancet
- Influenza Research Database – Database of influenza genomic sequences and related information.
- CDC 2009 H1N1 Influenza Vaccine Supply Status
- The H1N1 Pandemic and Global Health Security, Dean Julio Frenk, 2009-09-17
Europe
- Health-EU Portal EU response to influenza
- 2009 Pandemic Influenza A(H1N1) at the European Centre for Disease Prevention and Control (ECDC)
- European Commission – Public Health EU coordination on Pandemic (H1N1) 2009.
- UK National Pandemic Flu Service
- Official UK government information on swine flu from Directgov
- Official swine flu advice and latest information from the UK National Health Service
- Human/Swine A/H1N1 Influenza Origins and Evolution – Analysis of genetic data for the origin and evolution of swine flu virus.
North America
- Health Canada flu portal
- Pan-American Health Organization (PAHO) Swine Influenza portal
- H1N1 Influenza (Flu) portal at the US Centers for Disease Control (CDC)
- US Government swine, avian and pandemic flu portal
- Medical Encyclopedia Medline Plus: Swine Flu
- Swine Flu Outbreak, Influenza Virus Resource – Sequences and related resources (GenBank, NCBI)
India
|
|||||||||||||||||||||||||||||||
|
|||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
